Isolating unique cell populations for further experimental analyses can answer critical research questions in translational and clinical research – empowering observational and therapeutic studies. A popular approach is to isolate the heterogeneous population of cells in peripheral blood mononuclear cells (PBMCs) using the Ficoll method, followed by further purification to yield unique immune cell subsets, such as activated T cells for further downstream analyses. Specific cell types can also be isolated directly from whole peripheral blood, bone marrow, or cord blood. In general, there are two fundamental approaches to separate cells:
Positive cell selection: The desired cells in the sample are labeled so that they can be isolated into a separate fraction and the supernatant is discarded.
Negative cell selection: The unwanted cells in the sample are labeled to be isolated, allowing for the desired cells to be unlabeled and found in the supernatant.
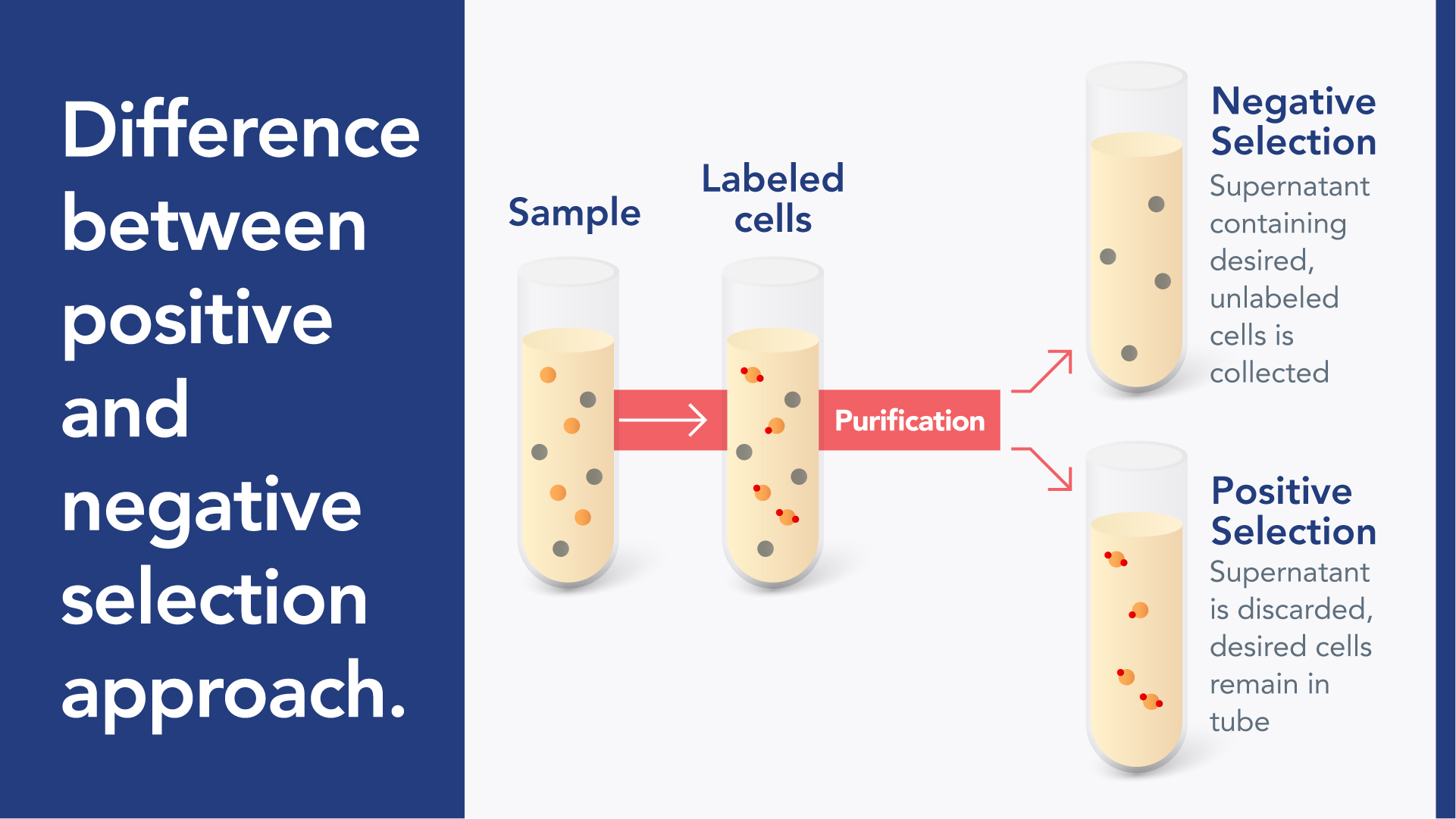
The difference between positive and negative selection approaches.
Selecting the best methodology for cell separation in experimental design requires thoughtful planning. There are many methods relying on positive or negative cell selection with benefits and drawbacks that can affect applicability in future experiments.
Some important aspects to consider are:
-
What are the downstream experiments planned?
The key consideration in deciding how and what cell types to isolate lies in the downstream functional and biological assays required in the study. Requiring only a certain activated T cell subpopulation will have greater demands than an indiscriminate requirement for just any PBMCs. It’s also important to decide if differentiation, genetic engineering, or cell expansion in tissue culture will be needed to characterize certain biomarkers in downstream molecular assays using antibodies, ELISAs, or gene expression profiling. Each type of approach has its own demands – most importantly centered upon purity, viability, and functionality of the sample. Tailoring the isolation technique to meet the demands of the study is the most important initial consideration.
-
What isolation technique is best suited for your needs?
-
Antibody-based approaches:
(1) Immunomagnetic cell separation harnesses magnetically-tagged antibodies against specific cell surface antigens to either select for (positive selection) or deplete (negative selection) specific cell types. A particular antibody binding to the cells of interest will retain them in the magnetic fraction and will require subsequent purification. If an antibody that binds to unwanted cells is used, the eluate will contain the desired cell fraction for further analyses.
(2) Fluorescence-activated cell sorting (FACS) uses fluorophore-tagged antibodies, and often several simultaneously, to sort cells individually into various subsets. FACS is advantageous because cells can be isolated based on intracellular markers (like GFP), in addition to typical surface biomarkers. It is the gold standard for specificity and diversity in cell populations isolated, however, it is also the most time-consuming and costly approach. -
Density-based centrifugation is the most commonly used, industry-standard method that relies on the various densities of cell types and a separation medium (such as Ficoll-Paque™) to sort a diverse collection of cell types, such as PBMCs. A variant of this approach, known as rosetting, uses antibodies against unwanted cell types causing them to form complexes with erythrocytes that can then be pelleted in a negative selection approach.
-
Adhesion: A simple, yet slow approach that relies on cellular adhesion properties to isolate cells of interest in culture. Collecting the supernatant of non-adhered cells allows for the isolation of suspension cells, whereas discarding the supernatant will yield only adhered cell types. Although cost-effective, this results in heterogeneous cell populations which can often be overshadowed by faster proliferating cells.
-
-
Should multiple isolation techniques be used?
Improved purification can be achieved through sequential methods that eliminate key problematic cell types easily and efficiently. This is particularly useful when a sample has a large number of unwanted cells (e.g., blood) in it and a pre-enrichment step would enhance subsequent separation methods. Often, this pre-enrichment step is done using density gradients or antibody-based negative selection to eliminate the majority of unwanted cell populations with ease. The purified population can then be further isolated into desired subsets using antibody-based approaches – reducing cell sorting time and increasing purity.
-
Importance of balancing purity, recovery, and yield
Generally, the higher the purity of cell subtypes required, the lower yield. If a large number of cells are needed for the study, a broader cell type selection is necessary. Asking for PBMCs, just T cells or only CD4+ T cells requires varying degrees of rigorous separation, with decreased yield as specificity increases. Density-based separation gives a heterogeneous cell population, so, to enrich for specific cell surface markers (like CD4+ cells), antibody-based purification is required. It’s important to understand all cell types that express certain biomarkers, as CD4+ cells could represent some regulatory T cells, T lymphocytes, and also monocytes.1 The highest purity is achieved through positive selection methods—often resulting in lower yields, but with less carryover of unwanted cell types.One key factor in purity and recovery relies on producing single-cell suspensions prior to positive or negative selection to reduce cell aggregation within samples. Aggregation can lead to reduced purity because either non-target cells can be co-isolated, or cells of interest can be lost to the unwanted fraction. Using fresh samples less than 24 hours post-collection results in higher purity of cell types isolated, because dead cells and other cell types can often contaminate desired fractions or release factors that can interfere with your analyses
-
Preserving function and viability
When it is critical to preserve cellular functionality, it is always better to use a negative selection approach, if possible, because this leaves the cell population in the supernatant unaffected by the method used to eliminate the other cell types. If positive selection is preferred, verifying that biomarker of interest and cell phenotypes will be unaffected by antibody binding and purification is crucial. Generally, cell viability after negative or positive selection remains comparable to the starting sample. Key determinants in viability, outside of starting sample quality, are based on speed and skill in performing the protocol. -
Are multiple isolated cell types desired from the same sample?
Although FACS sorting can yield the most diverse and reliable cell sorting, it can be cost-prohibitive and not necessary if just a few common cell types are desired. A sequential immunomagnetic approach combining both positive and negative selection strategies can be used. Sequentially separating each cell type from one sample, rather than fractionating it into separate aliquots, will increase yield and reduce loss due to low volumes or cell density. Positive selection can be performed several times but must only be done on an unlabelled cell population. It is usually best to start isolating the rarest or valuable cell type first and leave the most common cell types for last. Picking unique biomarkers for each desired cell type is critical to make sure there are no contaminating cells that may also express these markers.
-
Cost and automation
A reality that all clinical studies must balance is research funding limitations in experimental design. Utilizing the simplest and quickest method possible to isolate cells by evaluating what factors (e.g., yield, purity, functionality) are most important can help reduce costs, but should not come at the expense of confidently answering the research question. Although more costly, antibody-based approaches are the gold-standard and yield the highest purity. Immunomagnetic methods can also be scaled up to be automated to minimize sample handling—reducing cost while ensuring reproducibility. Automation is possible for simultaneous or sequential cell isolation and for virtually any cell type through positive or negative selection protocols. Often, common cell selection approaches for certain immune cell subsets are available as commercially validated, easy-to-use kits. Kits can ensure consistency and quality across samples processed at different times, and can often be automated to higher throughput.
Overall, several key considerations must be taken into account when deciding what cell separation methods to use in a clinical study. Minimizing interference from too many cell types can reduce experimental complexity and lend confidence in the data generated. Formulating the optimal experimental design can be pivotal in generating meaningful results and success in translational and clinical research studies.
References
1. Tomlinson MJ, Tomlinson S, Yang XB, Kirkham J. Cell separation: Terminology and practical considerations. J Tissue Eng. 2013;4:2041731412472690.